
Chemistry, 07.11.2019 06:31 YoVeoAnime
A1.50-liter sample of dry air in a cylinder exerts a pressure of 3.0 atmospheres at a temperature of 25°c. without change in temperature, a piston is moved in the cylinder until the pressure in the cylinder is reduced to 1.0 atmosphere. what is the volume of the gas? (be sure to use the correct number of significant figures.) 0.22 l 0.50 l 2.0 l 4.5 l

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 06:30, irvinbhangal2
What effect might melting sea ice have for people who live in coastal areas?
Answers: 1


Chemistry, 22.06.2019 10:00, Cythina2007
The reactions shown here can be combined to make the overall reaction c(s) + h2o(g) ⇌ co(g) + h2(g) by reversing some and/or dividing all the coefficients by a number. a. c(s) + o2(g) → co2(g) k=1.363×10^69 b. 2 h2(g) + o2(g) → 2 h2o(g) k=1.389×10^80 c. 2co(g) + o2 (g) → 2 co2(g) k=1.477×10^90
Answers: 1

Chemistry, 22.06.2019 15:30, 20cschultz
Which suspect most likely committed the robbery and how do you know
Answers: 2
You know the right answer?
A1.50-liter sample of dry air in a cylinder exerts a pressure of 3.0 atmospheres at a temperature of...
Questions in other subjects:

Mathematics, 07.01.2022 06:20

Mathematics, 07.01.2022 06:20




Social Studies, 07.01.2022 06:20

Social Studies, 07.01.2022 06:30


Arts, 07.01.2022 06:30

Biology, 07.01.2022 06:30

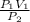
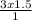 = 4.5L
= 4.5L

