
Chemistry, 15.10.2019 17:30 living8539
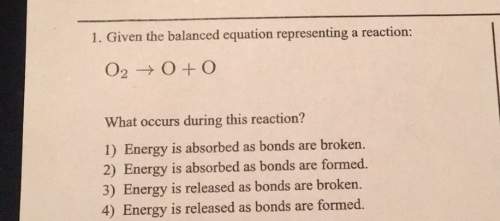
L. given the balanced equation representing a reaction: what occurs during this reaction? 1) energy is absorbed as bonds are broken.2) energy is absorbed as bonds are formed.3) energy is released as bonds are broken4) energy is released as bonds are formed.

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 15:30, kathleensumter4913
219 grams of iron (iii) oxide reacts with excess carbon according to the reaction equation shown below. fe2o3 + c → fe + co2 after a scientist performs the chemical reaction they find the actual yield of iron to be 57.4 grams. calculate the percent yield of this chemical reaction.
Answers: 1

Chemistry, 22.06.2019 02:00, officialgraciela67
What is the volume occupied by 10.0 dm3 of gas at standard pressure after it has been compressedat constant temputure to 500.0 kpa?
Answers: 1

Chemistry, 22.06.2019 02:30, ulilliareinhart2
The is a particle with one unit of positive charge a. proton b. positron c. electron d. nucleus awnser quick it is a important science test!
Answers: 2

You know the right answer?
L. given the balanced equation representing a reaction: what occurs during this reaction? 1) energy...
Questions in other subjects:


Geography, 20.09.2019 20:00


History, 20.09.2019 20:00

Physics, 20.09.2019 20:00


History, 20.09.2019 20:00


History, 20.09.2019 20:00



