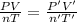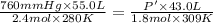Chemistry, 24.08.2019 09:50 greenbyron88
Initially, a 55.0 liter compressible container, holding 2.4 moles of a gas, exerts a pressure of 760 millimeters of mercury at a temperature of 280 kelvin. what is the pressure when the container is compressed to 43.0 liters, the moles of gas reduces to 1.8 moles, and the temperature changes to 36 degrees celsius?
93.7 mm hg
492 mm hg
740 mm hg
805 mm hg

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 12:50, martinez6221
What is the chemical name of the compound na2co3? use the list of polyatomic ions and the periodic table to you answer. a. sodium carbon oxide b. sodium carbonate c. sodium(ll) carbonate d. sodium oxalate
Answers: 1

Chemistry, 22.06.2019 16:30, jrfranckowiak
At 20°c, a sample of h2o liquid and a sample of co2 gas each have the same average kinetic energy. why is one a liquid and the other a gas at this temperature?
Answers: 1

Chemistry, 22.06.2019 22:00, aliciaa101
Ill give u brainliest pls how is mass of carbon conserved during cellular respiration
Answers: 1

Chemistry, 23.06.2019 06:40, Science2019
How many joules of heat are required to raise thetemperature of 750 g of water from 11.0 °c to 19.0 °c?
Answers: 1
You know the right answer?
Initially, a 55.0 liter compressible container, holding 2.4 moles of a gas, exerts a pressure of 760...
Questions in other subjects:

Mathematics, 01.07.2019 05:30

Mathematics, 01.07.2019 05:30


Biology, 01.07.2019 05:30

Social Studies, 01.07.2019 05:30





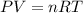
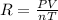 ...(1)
...(1)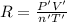 ..(2)
..(2)