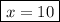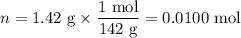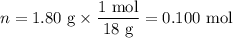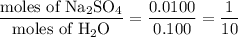Chemistry, 20.01.2020 08:31 mercedesamatap21hx0
When a 3.22 g sample of an unknown hydrate of sodium sulfate, na2so4 . x h2o (s), is heated, h2o (molar mass 18 g) is driven off. the mass of the anhydrous na2so4 (s) (molar mass 142 g) that remains is 1.42g. the value of x in the hydrate is

Answers: 3


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 06:00, jwood287375
When a spring is compressed, the energy changes from kinetic to potential. which best describes what is causing this change?
Answers: 3

Chemistry, 22.06.2019 06:00, josmanu235
Why is permeable soil best for plants that need a lot of drainage?
Answers: 1

Chemistry, 22.06.2019 18:00, AdoNice
Many pharmaceutical drugs are organic compounds that were originally synthesized in the laboratory. which two scientific disciplines are bridged by pharmaceutical drugs? chemistry and forensics chemistry and medicine biology and forensics biology and criminology
Answers: 2
You know the right answer?
When a 3.22 g sample of an unknown hydrate of sodium sulfate, na2so4 . x h2o (s), is heated, h2o (mo...
Questions in other subjects:

English, 08.09.2021 23:20



Chemistry, 08.09.2021 23:20


Mathematics, 08.09.2021 23:20


Mathematics, 08.09.2021 23:20

Advanced Placement (AP), 08.09.2021 23:20

Physics, 08.09.2021 23:20