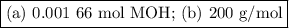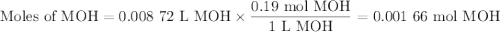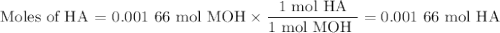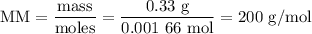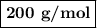Chemistry, 20.10.2019 06:50 goldenarrow
Using the concentration of the base and the volume of the base used, calculate the moles of the base used in the titration. then, using the mass of the acid, determine the molar mass of the acid.
data:
concentration of the base(naoh)= 0.19 m
volume of the base used= 8.72 ml
mass of the acid(unknown)= 0.33 g

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 18:00, ambarpena14
An object displaces 652 ml of water. the volume of the object is: 0.652 cm³ 6.52 cm³ 65.2 cm³ 652 cm³
Answers: 2

Chemistry, 22.06.2019 23:30, bxymichelle
With the largest atoms and the smallest number of valence electrons and with the smallest atoms and the greatest number of valence electrons are the most reactive. a. nonmetals; metals b. nonmetals; transition elements c. transition elements; metals d. metals; nonmetals
Answers: 3

Chemistry, 23.06.2019 02:00, Paytonsmommy09
Butane gas reacts with oxygen gas to give carbon dioxide gas and water vapor (gas). if you mix butane and oxygen in the correct stoichiometric ratio, and if the total pressure of the mixture is 390 mmhg, what is the pressure (in mmhg) of water vapor after the reaction is completed (temperature and volume do not change).
Answers: 2
You know the right answer?
Using the concentration of the base and the volume of the base used, calculate the moles of the base...
Questions in other subjects:



Chemistry, 29.07.2019 02:30




Mathematics, 29.07.2019 02:30



Mathematics, 29.07.2019 02:30