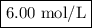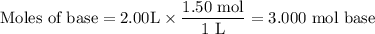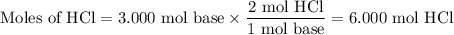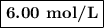Chemistry, 22.01.2020 21:31 sabahtramirez01
A1.00 l volume of hcl reacted completely with 2.00 l of 1.50 m ca(oh)2 according to the balanced chemical equation below. 2hcl + ca(oh)2 cacl2 + 2h2o what was the molarity of the hcl solution? 0.375 m 1.50 m 3.00 m 6.00 m

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 05:40, yah2muchh
Calculate: select the worksheet tab. this tab you calculate the analyte concentration. fill in the first set of boxes ("moles h2so4" and "moles naoh") based on the coefficients in the balanced equation. (if there is no coefficient, the value is 1.) record the appropriate volumes in the "ml naoh" and "ml h2so4" boxes. record the concentration of the titrant in the m naoh box. click calculate. what is the concentration listed
Answers: 2

Chemistry, 22.06.2019 15:30, dylannhandy
Using the first volume and temperature reading on the table as v1 and t1, solve for the unknown values in the table below. remember to use the rules of significant figures when entering your numeric response.
Answers: 2

Chemistry, 22.06.2019 17:10, glitterpanda2468
Calculate the estimated density of each ball. use the formula d = m/v where d is the density, m is the mass, and v is the volume. record your calculations in table a of your student guide. given that the density of water is 1.0 g/cm3, make a prediction about whether each ball will float in water. record your prediction in table a. what is the estimated density of the table tennis ball? record your answer to the nearest hundredth
Answers: 2
You know the right answer?
A1.00 l volume of hcl reacted completely with 2.00 l of 1.50 m ca(oh)2 according to the balanced che...
Questions in other subjects:

Mathematics, 08.06.2020 22:57

Mathematics, 08.06.2020 22:57

Mathematics, 08.06.2020 22:57

Mathematics, 08.06.2020 22:57

Mathematics, 08.06.2020 22:57

Biology, 08.06.2020 22:57

Mathematics, 08.06.2020 22:57

Mathematics, 08.06.2020 22:57

History, 08.06.2020 22:57

Spanish, 08.06.2020 22:57