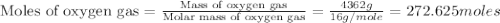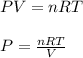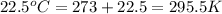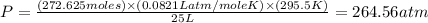Chemistry, 22.09.2019 19:20 glocurlsprinces
Using the ideal gas equation, calculate the pressure of oxygen gas in a cylinder with a volume of 25.00 l. the oxygen masses 4.362 kg and room temperature is at 22.5 o c. how many moles of oxygen are there and what is the pressure of oxygen in atmospheres in the cylinder according to the ideal gas law?

Answers: 2


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 14:00, cheyennemitchel238
Calculate the frequency of a wave in a spring toy. the wave has a speed of 1.1 meters per second and a wavelength of 0.1 meters. *
Answers: 2


Chemistry, 23.06.2019 02:00, hannabeth91
When an experimenter draws a conclusion that he assumes will apply to all situations set up similarly to his test situation, even though he cannot possibly have examined all possible test scenarios, the experimenter is using deductive reasoning inductive reasoning abductive reasoning subjective reasoning
Answers: 1
You know the right answer?
Using the ideal gas equation, calculate the pressure of oxygen gas in a cylinder with a volume of 25...
Questions in other subjects:

Mathematics, 23.01.2022 06:00








Mathematics, 23.01.2022 06:00

Mathematics, 23.01.2022 06:00