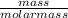Chemistry, 02.11.2019 23:31 pgjohnston001
A55.0g sample of iron (iii) filings is reacted with 23.8g of powdered sulfur (s8). how much iron (iii) sulfide in moles would be produced in this reaction?
equation:
convert to moles
of iron:
convert to moles
of sulfur:
calculate the
limiting reagent:
solve the problem:

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 17:00, pinkypie123457
What mass of carbon dioxide is produced from the complete combustion of 4.50×10−3 g of methane?
Answers: 2



Chemistry, 22.06.2019 11:50, robert7248
The chemical bond connecting one nucleotide with the next one along the nucleic acid chain is called a
Answers: 3
You know the right answer?
A55.0g sample of iron (iii) filings is reacted with 23.8g of powdered sulfur (s8). how much iron (ii...
Questions in other subjects:


Mathematics, 06.07.2019 09:30

Mathematics, 06.07.2019 09:30



Mathematics, 06.07.2019 09:30

History, 06.07.2019 09:30


History, 06.07.2019 09:30

History, 06.07.2019 09:30