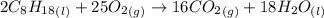Chemistry, 26.08.2019 21:00 ilovebeans25423
The combustion of octane, c8h18, proceeds according to the reaction
2c8h18(l)+25o2(> 16co2(g)+18h2o(l)
if 297 mol of octane combusts, what volume of carbon dioxide is produced at 20.0 °c and 0.995 atm?

Answers: 1


Other questions on the subject: Chemistry




Chemistry, 23.06.2019 06:30, morganzahn16
1.17 mol hcl and 2.5 mol naoh react according to the equation hcl + naoh -> nacl + h2o . if the limiting reactant is hcl, determine the amount of excess reactant that remains. answer in units of mol.
Answers: 1
You know the right answer?
The combustion of octane, c8h18, proceeds according to the reaction
2c8h18(l)+25o2(> 16co2(...
2c8h18(l)+25o2(> 16co2(...
Questions in other subjects:

Mathematics, 09.11.2021 22:00



Engineering, 09.11.2021 22:00

Mathematics, 09.11.2021 22:00

Chemistry, 09.11.2021 22:00