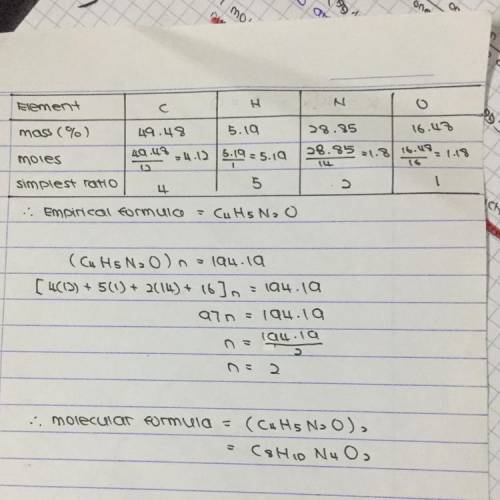
Chemistry, 03.02.2020 01:55 farhansayeed11
Caffeine has the following percent composition: carbon 49.48%, hydrogen 5.19%, oxygen 16.48% and nitrogen 28.85%. its molecular weight is 194.19 g/mol. what is its molecular formula?

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 05:00, mahhvelousg97
You mix the pks of succinic acid are 4.21 and 5.64. how many gramsa graduate student at sdsu wants to measure the activity of a particular enzyme at ph 4.0. to buffer her reaction, she will use a buffer system based on one of the acids listed below, which acid is most appropriate for the experiment? of monosodium succinate (fw = 140 g/mol) and disodium succinate (fw = 162 g/mol) must be added to 1 l of water to produce a solution with a ph 5.28 and a total solute concentration of 100 mm? (assume the total volume remains 1 liter, answer in grams monosodium succinate, grams disodium succinate, respectively.) volumes of 0.05 m nah2po4 and 0.05 m na2hpo4 (pk's for phosphoric acid are 2.15, 6.82 and 12.38). which of the following best describes the resulting solution?
Answers: 2

Chemistry, 22.06.2019 05:30, NorbxrtThaG
The table describes how some substances were formed substance 19 description formed by boiling pure water formed by combining three hydrogen atoms to every nitrogen atom formed by adding 5 g of sugar to 1 l of water formed by compressing carbon under high pressure based on the given descriptions, which substance is most likely a mixture?
Answers: 1
You know the right answer?
Caffeine has the following percent composition: carbon 49.48%, hydrogen 5.19%, oxygen 16.48% and ni...
Questions in other subjects:

Mathematics, 18.04.2020 00:05


Biology, 18.04.2020 00:05

History, 18.04.2020 00:05

History, 18.04.2020 00:05


Chemistry, 18.04.2020 00:05

Physics, 18.04.2020 00:05