
Chemistry, 04.02.2020 20:45 tyliyahmiles99
Calculate delta h in kj for the following reactions using heats of formation:
a) 2c2h6 (g) + 7o2 (g) > 4co2 (g) +6h2o (g)
b) 2pbo (s) + pbo2 (s) > pb3o4 (s)

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 06:00, Kjswagout5052
In an investigation that uses the scientific method, which step immediately follows making a hypothesis? o summarizing the results o asking a question o making observations designing an experiment mark this and retum save and exit next submit
Answers: 2

Chemistry, 22.06.2019 07:30, gwenparks
Calculate the ratio of h+ ions to oh– ions at a ph = 7. find the concentration of h+ ions to oh– ions listed in table b of your student guide. then divide the h+ concentration by the oh– concentration. record this calculated ratio in table a of your student guide. compare your approximated and calculated ratios of h+ ions to oh– ions at a ph = 7. are they the same? why or why not? record your comparison in table a. what is the concentration of h+ ions at a ph = 7? mol/l what is the concentration of oh– ions at a ph = 7? mol/l what is the ratio of h+ ions to oh– ions at a ph = 7? : 1
Answers: 1

Chemistry, 22.06.2019 13:00, torigirl4126
In what environment would mineral formation caused by high pressures and high temperatures most likely occur?
Answers: 3

Chemistry, 22.06.2019 20:00, batoolishak7475
Carbon-14 undergoes radioactive decay in the reaction above. determine the type of radiation emitted in this reaction and describe what is happening to the nucleus during this reaction.
Answers: 2
You know the right answer?
Calculate delta h in kj for the following reactions using heats of formation:
a) 2c2h6 (g) +...
a) 2c2h6 (g) +...
Questions in other subjects:





Chemistry, 08.01.2021 20:40

English, 08.01.2021 20:40

English, 08.01.2021 20:40

Mathematics, 08.01.2021 20:40

English, 08.01.2021 20:40

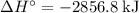 per mole reaction.
per mole reaction. per mole reaction.
per mole reaction.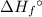 of a substance?
of a substance? 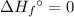 for the most stable allotrope of each element under standard conditions. For example, oxygen
for the most stable allotrope of each element under standard conditions. For example, oxygen 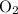 (not ozone
(not ozone 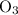 ) is the most stable allotrope of oxygen. Also, under STP
) is the most stable allotrope of oxygen. Also, under STP 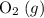 from itself does not involve any chemical or physical change. As a result,
from itself does not involve any chemical or physical change. As a result,  in particular) and the sign of the enthalpy changes.
in particular) and the sign of the enthalpy changes.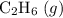 :
: 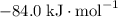 ;
;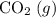 :
: 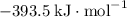 ;
;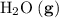 :
: 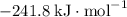 ;
;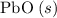 :
: 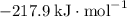 ;
;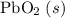 :
: 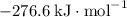 ;
;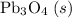 :
: 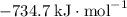
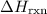 (or simply
(or simply 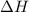 from enthalpies of formation?
from enthalpies of formation?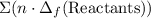 to show that this value takes the coefficients into account.Multiply the enthalpy of formation of each reactant by its coefficient in the equation.Find the sum of these values. Label the sum
to show that this value takes the coefficients into account.Multiply the enthalpy of formation of each reactant by its coefficient in the equation.Find the sum of these values. Label the sum 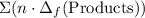 to show that this value takes the coefficient into account.Change = Final - Initial. So is the case with enthalpy changes.
to show that this value takes the coefficient into account.Change = Final - Initial. So is the case with enthalpy changes. 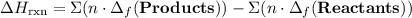 .
. 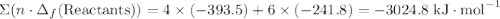 ;
;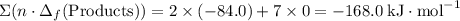 ;
;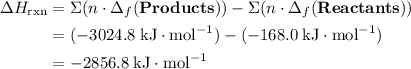 .
.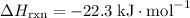 .
.

