

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 18:00, ReveenatheRaven2296
Which type of reaction always has an element and a compound as reactants
Answers: 1

Chemistry, 21.06.2019 20:30, pennygillbert
In which layer of earth do most earthauakes occur a_ inner core b_outer core c_mantle d_crust
Answers: 1

You know the right answer?
The reaction a + 2b + c -- > d + 2e is first order in reactant a, first order in b, and second or...
Questions in other subjects:





Mathematics, 02.03.2020 22:53




Arts, 02.03.2020 22:53

![\text{Rate}=k[A][B][C]^2](/tpl/images/0287/3858/2c5a0.png)
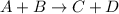
![\text{Rate}=k[A]^a[B]^b](/tpl/images/0287/3858/10aeb.png)
![[A]](/tpl/images/0287/3858/6aa06.png) and
and ![[B]](/tpl/images/0287/3858/db909.png) = concentration of A and B reactant
= concentration of A and B reactant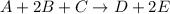
![\text{Rate}=k[A]^1[B]^1[C]^2](/tpl/images/0287/3858/d33f5.png)



