
One of the main components of an airbag is the gas that fills it. as part of the design process, you need to determine the exact amount of nitrogen that should be produced. calculate the number of moles of nitrogen required to fill the airbag. show your work. assume that the nitrogen produced by the chemical reaction is at a temperature of 495°c and that nitrogen gas behaves like an ideal gas. use this fact sheet to review the ideal gas law.

Answers: 2


Other questions on the subject: Chemistry



Chemistry, 22.06.2019 17:10, hahahwha
Acalorimeter is to be calibrated: 51.203 g of water at 55.2 degree c is added to a calorimeter containing 49.783 g of water at 23.5c. after stirring and waiting for the system to equilibrate, the final temperature reached is 37.6 degree c. specific heat capacity of water (s = 4.18 j/g∙degree c). calculate the calorimeter constant. (smδt)warm water = -[(smδt)cold water + (calorimeterδtcold water)]
Answers: 2

Chemistry, 22.06.2019 22:30, kristen17diaz
How many valence electrons are in atom of radon?
Answers: 1
You know the right answer?
One of the main components of an airbag is the gas that fills it. as part of the design process, you...
Questions in other subjects:

Mathematics, 27.11.2021 22:20

Mathematics, 27.11.2021 22:20



History, 27.11.2021 22:20


Physics, 27.11.2021 22:20

English, 27.11.2021 22:20

English, 27.11.2021 22:20

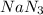 ,
, 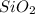 and
and 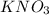 .The temperature at collision increases and
.The temperature at collision increases and 

