
Chemistry, 08.11.2019 02:31 pattydixon6
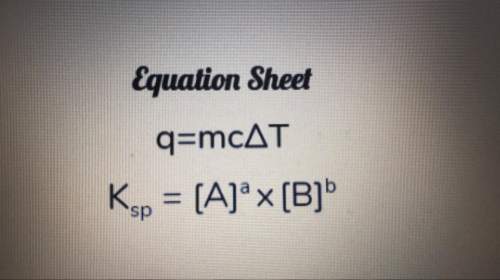
A3.50-g piece of metal is heated, and temperature of the metal changes from 17.0°c to 82.0°c. the amount of heat absorbed is 1435 j. what is the specific heat of the metal? show your work.

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 21:30, artemiscrock77041
The reaction q+r2=r2q is found to be first order in r2 and
Answers: 1


Chemistry, 22.06.2019 14:30, Tooey2331
1) describe the physical layout of the ocean floor ? 2) explain how the dumbo octopus swims differently than other octopus species and why this would be an advantage in the aphonic zone . 3) why are the types of organisms that live at each underwater hot vent so dramatically different ?
Answers: 3

Chemistry, 22.06.2019 18:00, heggestade
To apply in a gold the individual gold atoms are united to each other by means of a metallic bond. how would you use the gold block to determine the atomic radius of a gold atom?
Answers: 3
You know the right answer?
A3.50-g piece of metal is heated, and temperature of the metal changes from 17.0°c to 82.0°c. the am...
Questions in other subjects:


Mathematics, 04.08.2019 16:30

Spanish, 04.08.2019 16:30



Mathematics, 04.08.2019 16:30

Spanish, 04.08.2019 16:30


Mathematics, 04.08.2019 16:30

Mathematics, 04.08.2019 16:30



