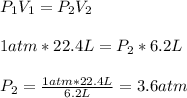Chemistry, 25.12.2019 21:31 lilmamaforev5093
22.4 liters of a gas at a constant temperature of zero celsius and a pressure of 1 atmosphere is compressed to a volume of 6.2 liters. what’s is the new pressure of the gas?

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 02:00, lwattsstudent
In the following redox reaction which is the oxidizing agent and which is the reducing agent? alcl3 + na nacl + al
Answers: 1

Chemistry, 22.06.2019 04:30, rosetoheart2
What are the three major branches of natural science? • earth and space science, life science, physical science •earth and space science, physical science, chemistry •physical science, life science, chemistry •life science, chemistry, physics
Answers: 1

Chemistry, 22.06.2019 09:00, SilverTheAmarok
This chart lists four kinds of polymers and their sources. what can be known about all four polymers, despite their differences? they come from living things. they share ionic carbon bonds. they are at least 100 monomers long. they are made of repeating subunits.
Answers: 2

Chemistry, 22.06.2019 11:30, claudr03
If we compare and contrast electromagnetic waves with sound waves, all but one statement is true. that is a) sound waves require a medium to travel while electromagnetic waves do not. b) electromagnetic waves can travel through the vacuum of space while sound waves cannot. c) electromagnetic waves must have a medium in which to travel, but sound waves can travel anywhere. eliminate d) sound waves must bounce off of matter in order to travel while electromagnetic waves do not require matter to be present.
Answers: 3
You know the right answer?
22.4 liters of a gas at a constant temperature of zero celsius and a pressure of 1 atmosphere is com...
Questions in other subjects:



Physics, 07.09.2021 02:50


Mathematics, 07.09.2021 02:50

Mathematics, 07.09.2021 02:50


Mathematics, 07.09.2021 02:50