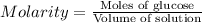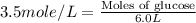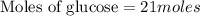21 moles

Chemistry, 10.10.2019 02:40 hunterl0513
How many moles of glucose (c6h12o6) are in 6.0 liters of a 3.5 m c6h12o6 solution?
21 moles
9.5 moles
3.5 moles
1.7 moles

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 16:50, briansalazar17
Answer asap need by wednesday morning calculate the ph of 0.16m ch3cooh which has ka = 1.74 x 10-5 mol dm-3 best answer will be brainliest
Answers: 3

You know the right answer?
How many moles of glucose (c6h12o6) are in 6.0 liters of a 3.5 m c6h12o6 solution?
21 moles
21 moles
Questions in other subjects:

History, 27.04.2021 22:00


Social Studies, 27.04.2021 22:00

Health, 27.04.2021 22:00

English, 27.04.2021 22:00

World Languages, 27.04.2021 22:00



Biology, 27.04.2021 22:00