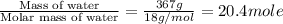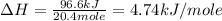Chemistry, 28.09.2019 22:00 emalvidrez5205
The initial temperature of the water in a constant-pressure calorimeter is 24°c. a reaction takes place in the calorimeter, and the temperature rises to 87°c. the calorimeter contains 367 g of water, which has a specific heat of 4.18 j/(g·°c). calculate the enthalpy change (δh) during this reaction

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 19:10, cristinaledford3696
Imagine that you have produced several versions of lactase, each of which differs from normal lactase by a single amino acid. describe a test that could indirectly determine which of the versions significantly alters the three-dimensional shape of the lactase protein.
Answers: 2


Chemistry, 22.06.2019 10:40, rntaran2002
What is the ph of a 0.0010 m hno3? 1.0 3.0 4.0 5.0
Answers: 2

Chemistry, 22.06.2019 17:00, calmicaela12s
Which statement is true about a catalyst? a: a catalyst decreases the rate of the reaction. b. a catalyst is consumed during a chemical reaction. c. a catalyst lowers the activation energy of a reaction. d. a catalyst increases the reactant concentration during a reaction.
Answers: 1
You know the right answer?
The initial temperature of the water in a constant-pressure calorimeter is 24°c. a reaction takes pl...
Questions in other subjects:



History, 28.08.2019 01:40

English, 28.08.2019 01:40



Biology, 28.08.2019 01:40

History, 28.08.2019 01:40


Mathematics, 28.08.2019 01:40

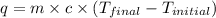
 = specific heat of water =
= specific heat of water = 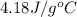
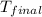 = final temperature of water =
= final temperature of water = 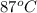
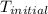 = initial temperature of metal =
= initial temperature of metal = 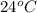
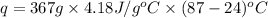
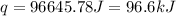
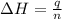
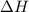 = enthalpy change = ?
= enthalpy change = ?