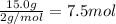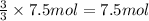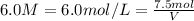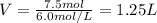Chemistry, 02.02.2020 16:52 dontcareanyonemo
What is the minimum amount of 6.0 m h2so4 necessary to produce 15.0 g of h2(g) according to the reaction? 2al(s)+3h2so4(aq)→al2(so4)3(aq)+3h2 (g)

Answers: 3


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 15:30, lovebaeforlife351
Each of the following reactions is allowed to come to equilibrium and then the volume is changed as indicated. predict the effect (shift right, shift left, or no effect) of the indicated volume change. drag the appropriate items to their respective bins. co(g) + h2o(g) < => co2(g) + h2(g) (volume is decreased) pcl3(g) + cl2(g) < => pcl5(g) (volume is increased) caco3(s)< => cao(s) + co2(g) (volume is increased)
Answers: 1

Chemistry, 22.06.2019 17:10, gungamer720
Increasing the substrate concentration in an enzymatic reaction could overcome which of the following? a) the need for a coenzymeb) allosteric inhibitionc) competitive inhibitiond) insufficient cofactors
Answers: 1

Chemistry, 22.06.2019 21:30, thompsonhomes1
If you burn 46.6 g of hydrogen and produce 416 g of water, how much oxygen reacted
Answers: 3
You know the right answer?
What is the minimum amount of 6.0 m h2so4 necessary to produce 15.0 g of h2(g) according to the reac...
Questions in other subjects:

World Languages, 26.01.2021 04:40


English, 26.01.2021 04:40






Social Studies, 26.01.2021 04:40

Mathematics, 26.01.2021 04:40