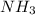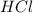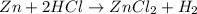Chemistry, 14.12.2019 18:31 larreanathalie3523
When zinc reacts with hydrochloric acid, it produces hydrogen gas. as the reaction proceeds, why does the rate of production of hydrogen gas decrease?
a. the concentration of hydrogen gas decreases.
b. the concentration of the reactants decreases.
c. the hydrogen gas formed inhibits the reaction.
d. the hydrogen gas also reacts with the zinc metal.

Answers: 1


Other questions on the subject: Chemistry


Chemistry, 23.06.2019 00:10, Rubendelarosa1529
Covalent compounds: mastery test select the correct answer what is formed when atoms join together with a covalent bond? a. an ion b. a molecule c. a neutral atom d. a noble gas
Answers: 3

Chemistry, 23.06.2019 04:31, 24swimdylanoh
What are the coefficients that will balance the skeleton equation below? n2 + h2 → nh3
Answers: 1
You know the right answer?
When zinc reacts with hydrochloric acid, it produces hydrogen gas. as the reaction proceeds, why doe...
Questions in other subjects:

Biology, 15.07.2019 16:30

English, 15.07.2019 16:30

Biology, 15.07.2019 16:30



Mathematics, 15.07.2019 16:30


Mathematics, 15.07.2019 16:30

Health, 15.07.2019 16:30

![Rate=k[NH_3]^x[HCl]^y](/tpl/images/0418/8412/2eb4c.png)