
Chemistry, 03.11.2019 07:31 twalters88
Read the given equation. 2na + 2h2o → 2naoh + h2
during a laboratory experiment, a certain quantity of sodium metal reacted with water to produce sodium hydroxide and hydrogen gas. what was the initial quantity of sodium metal used if 6.30 liters of h2 gas were produced at stp?
a.) 10.3 grams
b.) 12.9 grams
c.) 14.7 grams
d.) 15.2 grams

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 00:00, tahjaybenloss16
The pressure inside a hydrogen-filled container was 2.10 atm at 21 ? c. what would the pressure be if the container was heated to 92 ? c ?
Answers: 2


Chemistry, 22.06.2019 07:20, camillexv2668
2pos suppose an object in free fall is dropped from a building. its starting velocity is 0 m/s. ignoring the effects of air resistance, what is the speed (in m/s) of the object after falling 3 seconds? give your answer as a positive decimal without units. answer here
Answers: 1

Chemistry, 22.06.2019 10:30, perezanthony2403
Which describes fat? a: a carbohydrate that produces energy b: a nucleic acid that directs cell function c: a lipid that stores energy d: a protein that speeds up a chemical reaction
Answers: 1
You know the right answer?
Read the given equation. 2na + 2h2o → 2naoh + h2
during a laboratory experiment, a certain qu...
during a laboratory experiment, a certain qu...
Questions in other subjects:


History, 05.07.2019 15:30



Health, 05.07.2019 15:30


Mathematics, 05.07.2019 15:30

History, 05.07.2019 15:30


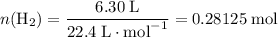 .
.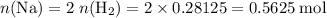 .
.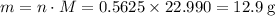 .
.

