Caco3(s) ∆→cao(s) + co2(g).
if 13.2 g of co2 was produced from the thermal decomposition of 40...


Answers: 2


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 19:40, raquelqueengucci25
In this synthesis reaction what products will form
Answers: 1

Chemistry, 22.06.2019 10:40, justicejesusfreak
Which buffer would be better able to hold a steady ph on the addition of strong acid, buffer 1 or buffer 2? explain. buffer 1: a solution containing 0.10 m nh4cl and 1 m nh3. buffer 2: a solution containing 1 m nh4cl and 0.10 m nh3
Answers: 1

Chemistry, 22.06.2019 13:20, alejandra340
Can someone me with 3 and 4 plz. this is for masteries test.
Answers: 2

Chemistry, 23.06.2019 00:30, Keemdadream13
If there are 3.5 moles of koh, how many moles of naoh can be produced? question 1 options: a)3.0 moles naoh b)3.5 moles naoh c)1 moles naoh d)9 moles naoh
Answers: 1
You know the right answer?
Questions in other subjects:


Computers and Technology, 01.12.2021 02:10

Computers and Technology, 01.12.2021 02:10





Mathematics, 01.12.2021 02:10


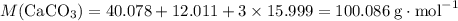 .
.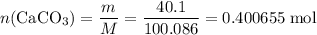 .
.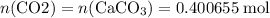 .
.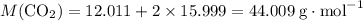 .
. expected for the 40.1 grams of CaCO₃:
expected for the 40.1 grams of CaCO₃: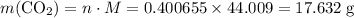 .
. .
.

