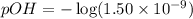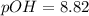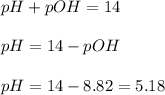0.176

Chemistry, 17.01.2020 09:31 Angelanova69134
What is the ph of a solution with a 1.50 × 10−9 m hydroxide ion concentration?
0.176
5.18
8.82
9.20

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 19:00, cutebab4786
Consider the point on the plot where 10.0 g of naoh have been added. what amount of naoh, in moles, has been added? 0.308 mol fecl3 initially present
Answers: 1

Chemistry, 22.06.2019 04:50, aletadaboss
Acompound contains c, h, and o atoms. when 1.130 g of the compound is burned in oxygen, 1.064 g co2 and 0.3631 g h2o are produced. what is the empirical formula of this compound?
Answers: 1


Chemistry, 22.06.2019 10:30, cheyennecarrillo14
If you add 5.00 ml of 0.100 m sodium hydroxide to 50.0 ml of acetate buffer that is 0.100 m in both acetic acid and sodium acetate, what is the ph of the resulting solution? acetic acid: ka = 1.8. x 10-5
Answers: 1
You know the right answer?
What is the ph of a solution with a 1.50 × 10−9 m hydroxide ion concentration?
0.176
0.176
Questions in other subjects:

Health, 19.11.2020 18:40



English, 19.11.2020 18:40

English, 19.11.2020 18:40

Mathematics, 19.11.2020 18:40


History, 19.11.2020 18:40


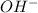 ion =
ion = 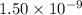
![pOH=-\log [OH^-]](/tpl/images/0459/2234/1fac1.png)