
Chemistry, 22.01.2020 18:31 adrian1742
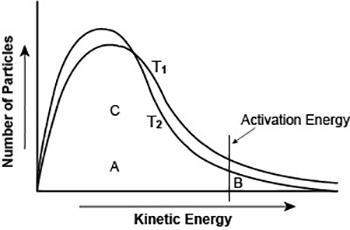
The graph shows the distribution of energy in the particles of two gas samples at different temperatures, t1 and t2. a, b, and c represent individual particles.
based on the graph, which of the following statements is likely to be true?
particle b is more likely to participate in the reaction than particle a.
particle c is more likely to participate in the reaction than particle b.
most of the gas particles have either very high or very low kinetic energies.
more gas particles participate in the reaction at t2 than at t1.

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 23:00, brapmaster764
What is the formula that this ionic compounds could form sr2+p3-o2-
Answers: 3

Chemistry, 22.06.2019 17:00, smelcher3900
According to the kinetic-molecular theory, what happens to a liquid when it is transferred from one container to another? the volume and the shape stay the same. the volume increases to fill the new container, but the shape stays the same. the volume stays the same, but the shape changes to fit the new container. the volume and the shape change to fill the new container.
Answers: 2


Chemistry, 23.06.2019 02:00, raulflores01
Which best describes the present-day universe? opaque, expanding very slowly, stars produce heavy elements transparent, expanding at an accelerated rate, stars produce heavy elements opaque, expanding at an accelerated rate, stars produce only hydrogen and helium transparent, expanding very slowly, stars produce only hydrogen and helium
Answers: 1
You know the right answer?
The graph shows the distribution of energy in the particles of two gas samples at different temperat...
Questions in other subjects:



English, 29.01.2020 04:55

Mathematics, 29.01.2020 04:55





Mathematics, 29.01.2020 04:55

Mathematics, 29.01.2020 04:55



