
consider the substances below.
• 1 mol of beryllium
• 1 mol of salt
• 1 mol of water
• 1 mol of hydrogen
which statement is true about these substances? 1) they have exactly the same mass.2) they have different numbers of particles.3) they have the same number of atoms.4) they have different masses.

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 19:30, umimgoingtofail
Motivation cannot be developed with practice; a person either possesses it or they do not.
Answers: 1


Chemistry, 22.06.2019 11:10, hannah2757
Which of the following shapes would represent a molecule with two bonded atoms and 3 lone pairs on only one of them , trigonal planar , bent , trigonal pyramidal , linear
Answers: 1
You know the right answer?
consider the substances below.
• 1 mol of beryllium
• 1 mol of salt
• 1 mol of water<...
• 1 mol of beryllium
• 1 mol of salt
• 1 mol of water<...
Questions in other subjects:




Mathematics, 02.05.2021 20:40



Mathematics, 02.05.2021 20:40

History, 02.05.2021 20:40


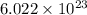 number of particles.
number of particles.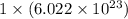 number of atoms.
number of atoms.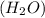 has 3 number of atoms. So, 1 mole of water contains
has 3 number of atoms. So, 1 mole of water contains 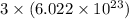 number of atoms.
number of atoms.

