
Chemistry, 08.10.2019 02:30 polyanskiymichael
Read the chemical equation. n2 + 3h2 → 2nh3 using the volume ratio, determine how many liters of nh3 is produced if 1.2 liters of h2 reacts with an excess of n2, if all measurements are taken at the same temperature and pressure? 1.8 liters 1.5 liters 0.90 liters 0.80 liters

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 01:30, kayleg907436
Arollercoaster car at the top of a hill has potential energy kinetic energy chemical energy light energy
Answers: 1

Chemistry, 22.06.2019 18:30, bibiansolis
The table lists the lattice energies of some compounds. compoundlattice energy (kj/mol)lif –1,036licl –853naf –923kf –821nacl –786which statement about crystal lattice energy is best supported by the information in the table? the lattice energy increases as cations get smaller, as shown by lif and kf. the lattice energy increases as the cations get larger, as shown by lif and licl. the lattice energy decreases as cations get smaller, as shown by nacl and naf. the lattice energy decreases as the cations get smaller, as shown by naf and kf.
Answers: 3

Chemistry, 22.06.2019 23:00, hailey5campbelp7d1c0
In which region is the substance in both the solid phase and the liquid phase? 1 2. 3 4 mark this and return save and exit next
Answers: 2

Chemistry, 23.06.2019 02:00, matthewsorrow02
What is the mass of 0.750 mole of aluminum oxide, al2o3?
Answers: 1
You know the right answer?
Read the chemical equation. n2 + 3h2 → 2nh3 using the volume ratio, determine how many liters of nh3...
Questions in other subjects:

Mathematics, 04.08.2019 12:00



Biology, 04.08.2019 12:00

Mathematics, 04.08.2019 12:00


Mathematics, 04.08.2019 12:00

Biology, 04.08.2019 12:00


 produced will be, 0.8 liters.
produced will be, 0.8 liters.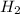 = 1.2 L
= 1.2 L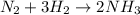
 volume of
volume of 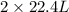 volume of
volume of 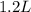 volume of
volume of 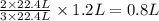 volume of
volume of 


