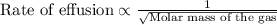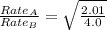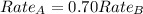Chemistry, 29.09.2019 01:30 zmoore3793
The molar mass of two equally sized samples of unknown gaseous compounds is shown in the table. molar mass comparison gas molar mass a 4.00 g/mol b 2.01 g/mol which statement describes the density and effusion of both gases at stp? gas a has a higher density and effuses faster than gas b. gas a has a higher density and effuses slower than gas b. gas a has a lower density and effuses faster than gas b. gas a has a lower density and effuses slower than gas b.

Answers: 2


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 08:40, jeffcarpenter
What is the value of keq for the reaction expressed in scientific notation?
Answers: 1

Chemistry, 22.06.2019 11:30, elijah1090
Aperfume bottle is dropped in the corner of a room. the odor of the perfume can be detected on the other side of the room. which statement best describes this observation?
Answers: 2

Chemistry, 22.06.2019 16:30, sbush1412
Ammonium perchlorate nh4clo4 is the solid rocket fuel used by the u. s. space shuttle. it reacts with itself to produce nitrogen gas n2 , chlorine gas cl2 , oxygen gas o2 , water h2o , and a great deal of energy. what mass of nitrogen gas is produced by the reaction of 2.1g of ammonium perchlorate?
Answers: 2
You know the right answer?
The molar mass of two equally sized samples of unknown gaseous compounds is shown in the table. mola...
Questions in other subjects:

Biology, 04.06.2021 19:20

Mathematics, 04.06.2021 19:20

Mathematics, 04.06.2021 19:20


Mathematics, 04.06.2021 19:20

English, 04.06.2021 19:20

Mathematics, 04.06.2021 19:30


History, 04.06.2021 19:30

Mathematics, 04.06.2021 19:30