
Chemistry, 02.10.2019 01:00 divagothboi
During an exothermic reaction, δh for the reactants was 890 kj/mol. which of the following statements is correct about the δh for the products?
a. it is less than 890 kj/mol because the amount of energy required to break bonds is less than the amount of energy released in forming bonds.
b. it is less than 890 kj/mol because the amount of energy required to break bonds is greater than the amount of energy released in forming bonds.
c. it is greater than 890 kj/mol because the amount of energy required to break bonds is less than the amount of energy released in forming bonds.
d. it is greater than 890 kj/mol because the amount of energy required to break bonds is greater than the amount of energy released in forming bonds.

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 04:30, coryoddoc3685
Turbo the snail moves across the ground at a pace of 12 feet per day. if the garden is 48 feet away, how many days will it take for the snail to get there?
Answers: 2

Chemistry, 22.06.2019 14:30, joejoefofana
Consider the reduction reactions and their equilibrium constants. cu+(aq)+e−↽−−⇀cu(s)pb2+(aq)+2e−↽−−⇀ pb(s)fe3+(aq)+3e−↽−−⇀fe(=6.2×108=4. 0×10−5=9.3×10−3 cu + ( aq ) + e − ↽ − − ⇀ cu ( s ) k =6.2× 10 8 pb 2 + ( aq ) +2 e − ↽ − − ⇀ pb ( s ) k =4.0× 10 − 5 fe 3 + ( aq ) +3 e − ↽ − − ⇀ fe ( s ) k =9.3× 10 − 3 arrange these ions from strongest to weakest oxidizing agent.
Answers: 3

You know the right answer?
During an exothermic reaction, δh for the reactants was 890 kj/mol. which of the following statement...
Questions in other subjects:

Mathematics, 28.08.2019 17:30

Physics, 28.08.2019 17:30

English, 28.08.2019 17:30


History, 28.08.2019 17:30



Mathematics, 28.08.2019 17:30

Mathematics, 28.08.2019 17:30

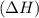 comes out to be positive.
comes out to be positive.

