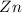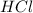When zinc reacts with hydrochloric acid, it produces hydrogen gas. as the reaction proceeds, why does the rate of production of hydrogen gas decrease? the concentration of hydrogen gas decreases. the concentration of the reactants decreases. the hydrogen gas formed inhibits the reaction. the hydrogen gas also reacts with the zinc metal.

Answers: 3


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 07:10, angellong94
Provide a stepwise curved arrow mechanism that fully explains the outcome of the reaction shown below. oh нао* heat он
Answers: 2

Chemistry, 22.06.2019 13:50, hannahmyung1113
Amap that uses a range of colors and shading to represent the elevation, depth, or landscape of specific features on earth is a/an map.
Answers: 3

Chemistry, 22.06.2019 15:20, mydoggy152
Fossil fuels are organic compounds that are made from
Answers: 1
You know the right answer?
When zinc reacts with hydrochloric acid, it produces hydrogen gas. as the reaction proceeds, why doe...
Questions in other subjects:

Health, 23.05.2021 01:00





Mathematics, 23.05.2021 01:00

Business, 23.05.2021 01:00


Mathematics, 23.05.2021 01:00

![Rate=k[A]^x[B]^y](/tpl/images/0383/1895/ddde1.png)