An aqueous solution is 15.0% by mass of copper(ii) sulfate pentahydrate, cuso4∙5h2o. what
is th...


Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 08:30, ebigham5117
If i initially have a gas at a pressure of 12 atm, a volume of 23 liters, and a temperature of 200 k, and then i raise the pressure to 14 atm and increase the temperature to 300 k, what is the new volume of the gas?
Answers: 2

Chemistry, 22.06.2019 09:30, mazielynn84
In 2002, the rare earth elements mine in mountain pass, california was closed because
Answers: 1

Chemistry, 22.06.2019 16:10, nauticatyson9
Given the following equation: 2a1 + 3mgcl2 --> 2alcl3 + 3mg how many moles of aluminum chloride are produced from 2.5 moles of magnesium chloride?
Answers: 1
You know the right answer?
Questions in other subjects:





Mathematics, 29.11.2021 21:20

Chemistry, 29.11.2021 21:20


English, 29.11.2021 21:20


Mathematics, 29.11.2021 21:20

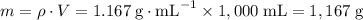 .
.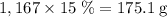 among that 1,167 grams of the solution is
among that 1,167 grams of the solution is 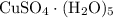 .
. 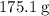 of the hydrate:
of the hydrate: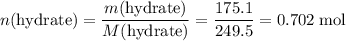 .
.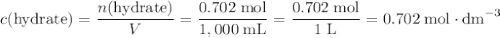 .
.

