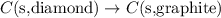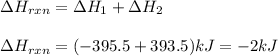Chemistry, 11.01.2020 03:31 mallorybranham
Given the equations below, which description applies to the conversion of diamond to graphite? c(s, diamond) + o2 (g) → co2 (g), ∆h = –395.4 kj co2 (g) → c(s, graphite) + o2 (g), ∆h = 393.5 kj explain your answer
a) energy is created during the process.
b) heat is neither released nor absorbed during the process.
c) heat is released during the process.
d) the process is endothermic.

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 01:40, georgehall3027
C3h8o3 - glycerol major species present when dissolved in water
Answers: 2

Chemistry, 22.06.2019 04:00, clairebear66
What three natural resources are found in the great lakes region
Answers: 2

Chemistry, 22.06.2019 09:00, heids17043
Chen drew a diagram to compare the ways in which different organisms obtain nitrogen. which label belongs to the area marked z?
Answers: 3

Chemistry, 22.06.2019 10:20, blondielocks2002
Gwhich r group would most likely be found in a hydrophobic area of the tertiary structure of a globular protein? which r group would most likely be found in a hydrophobic area of the tertiary structure of a globular protein? −ch2−oh −ch2−o||c−nh2 −ch2−coo− −ch2−ch2−ch2−ch2−n+h3
Answers: 3
You know the right answer?
Given the equations below, which description applies to the conversion of diamond to graphite? c(s,...
Questions in other subjects:










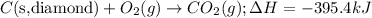 ....(1)
....(1)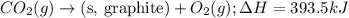 ....(2)
....(2)