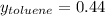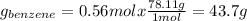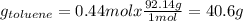Chemistry, 14.01.2020 15:31 citlalli30
Asolution of benzene (c6h6) and toluene (c7h8) is 25.0% benzene by mass. the vapor pressures of pure benzene and pure toluene at 25°c are 94.2 torr and 28.4 torr, respectively. assuming ideal behavior, calculate the following: (a) the vapor pressure of each solution component in the mixture (b) the total pressure above the solution (c) the composition of the vapor in mass percent why is the composition of the vapor different from the composition of the solution?

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 17:10, ladypink94
Abullet found at a crime scene may be used as evidence in a trial if the percentage of metals match to the composition of metals in a bullet from the suspect's ammunition. a forensic scientist's analysis of the bullet shows that it contains 11.9 g of lead, 0.5 g of tin, and 0.8 b of antimony. what is the percentage of lead metal in the bullet? express your answers to the one's place.
Answers: 2


Chemistry, 22.06.2019 05:20, anggar20
Why does the sun appear to be the brightest star in the sky? a- its apparent brightness is much greater than other stars. b- it burns more gas, making it brighter than any other star. c- it is the largest star in the galaxy, so it is the brightest star. d- its relative distance to earth is closer than the other stars.
Answers: 1

Chemistry, 22.06.2019 12:00, BakerElsie02
Which of the following units is not an official si unit? mole liter kilogram ampere
Answers: 1
You know the right answer?
Asolution of benzene (c6h6) and toluene (c7h8) is 25.0% benzene by mass. the vapor pressures of pure...
Questions in other subjects:


English, 05.09.2020 19:01


Computers and Technology, 05.09.2020 19:01

Biology, 05.09.2020 19:01





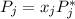
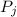 is the partial pressure of component j in the gas mixture above the solution,
is the partial pressure of component j in the gas mixture above the solution,  is the vapor pressure of the pure component and
is the vapor pressure of the pure component and 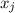 is the molar fraction of component j in the liquid mixture (in the solution).
is the molar fraction of component j in the liquid mixture (in the solution).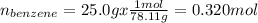

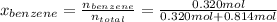 →
→ 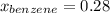
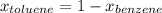 →
→ 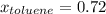

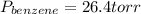
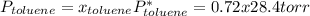

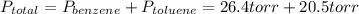


 is the mole fraction of the gas j in the gas mixture and
is the mole fraction of the gas j in the gas mixture and 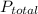 is the total pressure.
is the total pressure.

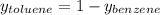 →
→