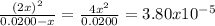The dissociation of molecular iodine into iodine atoms is represented as i2(g) ⇌ 2i(g) at 1000 k, the equilibrium constant kc for the reaction is 3.80 × 10−5. suppose you start with 0.0456 mol of i2 in a 2.28−l flask at 1000 k. what are the concentrations of the gases at equilibrium? what is the equilibrium concentration of i2

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 18:10, hannacarroll2539
The enthalpy of formation of water is -285.8 kj/mol. what can be inferred from this statement?
Answers: 1

Chemistry, 22.06.2019 14:30, davidrodriguez122001
Which of the following describes a situation where competition between producers exists
Answers: 1


Chemistry, 22.06.2019 19:00, QuestionsAnsweredNow
Suppose that a certain fortunate person has a net worth of $71.0 billion ($7.10×1010). if her stock has a good year and gains $3.20 billion (3.20×109) in value, what is her new net worth?
Answers: 3
You know the right answer?
The dissociation of molecular iodine into iodine atoms is represented as i2(g) ⇌ 2i(g) at 1000 k, th...
Questions in other subjects:

History, 01.10.2019 14:10


Mathematics, 01.10.2019 14:10



Biology, 01.10.2019 14:10

Mathematics, 01.10.2019 14:10



![\frac{[I]^{2} }{[I_{2} ]}](/tpl/images/0472/7611/f811c.png)
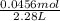 → [I₂]₀ = 0.0200 M
→ [I₂]₀ = 0.0200 M![\frac{[I]^{2} }{[I_{2} ]} = \frac{(2x)^{2} }{0.0200 - x} = 3.80x10^{-5}](/tpl/images/0472/7611/3afcc.png)