
Chemistry, 28.01.2020 07:31 squiddddplop
Plz answer concentration of either the h+ ion or the oh − ion is given for four aqueous solutions at 298 k.?
for each solution, calculate [h+] or [oh − ]. state whether the solution is acidic, basic, or neutral.
(a) [h+] = 8.0 * 10^-13 m
[oh − ] =
(b) [oh − ] = 4.0 * 10^-7 m
[h+] =
(c) [oh − ] = 5.0 * 10^-3 m
[h+] =
(d) [h+] = 8.0 * 10^-5 m
[oh − ] =

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 11:00, bigwaYne
Imagine that twenty i. u.’s of enzyme z were catalyzing the above reaction for one minute, under vmaxconditions, in a 3.00 ml assay volume. the assay is buffered with 20 mm phosphate buffer, ph 7.60. what will the ph be at the end of that one minute?
Answers: 2


Chemistry, 23.06.2019 08:50, leah5981
Reacting masses1 calcium carbonate breaks down on heating to produce calcium oxide and carbondioxide gas. caco3 + cao + co2a student heats 15 g of calcium carbonate strongly in a crucible. relative atomic masses (a): ca = 40, c = 12, o = 16.calculate the mass of calcium oxide produced by this reaction.(5 marks)
Answers: 3
You know the right answer?
Plz answer concentration of either the h+ ion or the oh − ion is given for four aqueous solutions a...
Questions in other subjects:

Biology, 15.12.2020 22:50

Chemistry, 15.12.2020 22:50



History, 15.12.2020 22:50

English, 15.12.2020 22:50


Mathematics, 15.12.2020 22:50


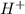 =
= 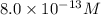
![[H^+][OH^-]=10^{-14}](/tpl/images/0475/8440/7d81f.png)
![(8.0\times 10^{-13})\times [OH^-]=10^{-14}](/tpl/images/0475/8440/5e33b.png)
![[OH^-]=1.25\times 10^{-2}M](/tpl/images/0475/8440/54850.png)
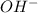 =
= 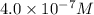
![[H^+]\times (4.0\times 10^{-7})=10^{-14}](/tpl/images/0475/8440/9d559.png)
![[H^+]=0.25\times 10^{-7}M](/tpl/images/0475/8440/b9c16.png)
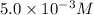
![[H^+]\times (3.0\times 10^{-3})=10^{-14}](/tpl/images/0475/8440/f3c6a.png)
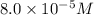
![(8.0\times 10^{-5})\times [OH^-]=10^{-14}](/tpl/images/0475/8440/87df8.png)


