
Chemistry, 30.01.2020 17:03 Isaiahtate053
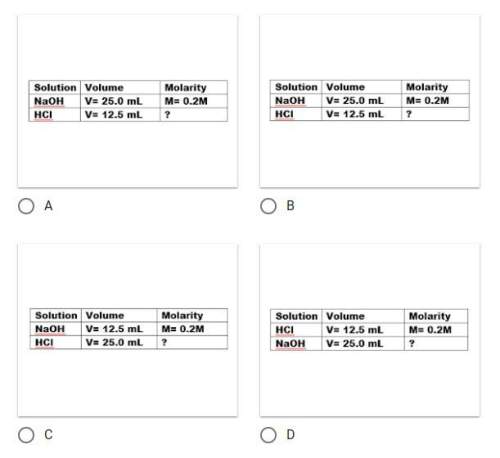
You have 25.0ml hcl of unknown concentration. it takes 12.5 ml of 0.2 m naoh to neutralize the acid. determine the concentration of hcl in the following steps.
which data table would you use to organize the information correctly?

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 07:00, coolkid2041
Calculate the number of moles of ethane in 100 grams
Answers: 3



Chemistry, 23.06.2019 23:50, SoccerHalo
In the following reaction, oxygen is the excess reactant. sicl4 + o2 → sio2 + cl2 the table shows an experimental record for the above reaction. experimental record trial starting amount of sicl4 starting amount of o2 actual yield of sio2 1 120 g 240 g 38.2 g 2 75 g 50 g 25.2 g part 1: calculate the percentage yield for sio2 for trial 1. also, determine the leftover reactant for the trial. show your work. part 2: based on the percentage yield in trial 2, explain what ratio of reactants is more efficient for the given reaction.
Answers: 3
You know the right answer?
You have 25.0ml hcl of unknown concentration. it takes 12.5 ml of 0.2 m naoh to neutralize the acid....
Questions in other subjects:

Mathematics, 19.03.2020 08:58

Mathematics, 19.03.2020 08:58


Physics, 19.03.2020 08:58



Mathematics, 19.03.2020 08:58






