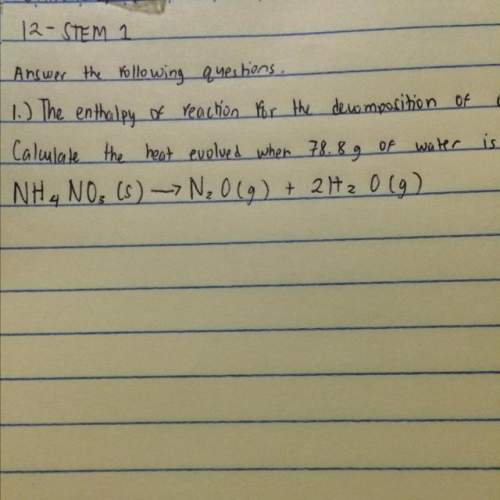Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 09:00, mercymain1014
An excess of lithium oxide undergoes a synthesis reaction with water to produce lithium hydroxide li2o+h2o→2lioh if 1.05 g of water reacted, what is the theoretical yield of lithium hydroxide? a) 5.83 x 10–2 g lioh b) 1.17 x 10–1 g lioh c) 2.79 x 100 g lioh d) 1.40 x 100 g lioh
Answers: 1


Chemistry, 22.06.2019 10:10, andersonemma2222
How do you identify the anode on a power source such as a battery? how do you identify the cathode? how are terms anion and cation?
Answers: 1

Chemistry, 22.06.2019 20:00, Chynadoll94
Iam hoping to create 5.72 grams of glucose. the plant was given 4.75 liters of co2 and 2.81 g of h20. which reactant was the limiting reagent? how much excess mass did we have of the other reactant?
Answers: 1
You know the right answer?
The enthalpy of reaction for the decomposition of ammonium nitrate, nh4no3 is -77.4 kj mol^-1. calcu...
Questions in other subjects:




Geography, 21.08.2019 04:00

History, 21.08.2019 04:00



History, 21.08.2019 04:00