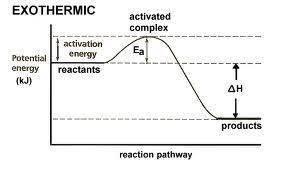
Which statement is true about the potential energy diagram for an exothermic reaction? the products have less potential energy than reactants. the energy value remains the same throughout the diagram. the graph starts at a lower energy value and ends at a higher energy value. the potential energy of the products is equal to the potential energy of the reactants

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 04:00, lucasrandall
Asolution contains 225 g of sodium chloride, nacl, dissolved in enough water to make a 0.25 l of solution. what is the molarity of the solution?
Answers: 2


Chemistry, 22.06.2019 06:00, kylieweeks052704
Which of the following did jj thompson discover about atoms? a)an atom has an internal structure. b) atoms are tiny indivisible particles. c)electrons orbit the nucleus of an atom. d) the nucleus of an atom contains protons and neutrons.
Answers: 2

Chemistry, 22.06.2019 11:00, peternice2956
Ais a mountain created from eruptions of lava, ash, rocks, and hot gases.
Answers: 1
You know the right answer?
Which statement is true about the potential energy diagram for an exothermic reaction? the products...
Questions in other subjects:

World Languages, 08.04.2021 17:20

Mathematics, 08.04.2021 17:20







Physics, 08.04.2021 17:20

Mathematics, 08.04.2021 17:20