
Chemistry, 21.11.2019 09:31 rubianny03
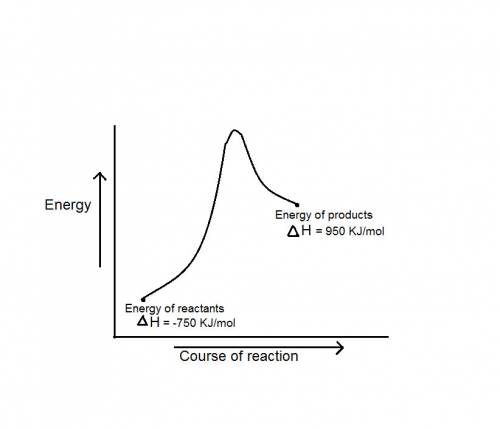
during a reaction, δh for reactants is −750 kj/mol and δh for products is 920 kj/mol. which statement is correct about the reaction?
it is endothermic because the energy required to break bonds in the reactants is less than the energy released when the products are formed.
it is endothermic because the energy required to break bonds in the reactants is greater than the energy released when the products are formed.
it is exothermic because the energy required to break bonds in the reactants is less than the energy released when the products are formed.
it is exothermic because the energy required to break bonds in the reactants is greater than the energy released when the products are formed.

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 13:00, froyg1234
Read the given expression. x = number of protons − number of core electrons which of the following explains the identity of x and its trends across a period? x is the effective nuclear charge, and it remains constant across a period. x is the screening constant, and it remains constant across a period. x is the effective nuclear charge, and it increases across a period. x is the screening constant, and it increases across a period.
Answers: 1

Chemistry, 21.06.2019 20:00, lorenaandreahjimenez
The answer for #3 is c but i don't know why
Answers: 1

Chemistry, 22.06.2019 03:00, HHHHHHHHHMMMMMMMMM
About 70 percent of the earth's surface is water-covered, and about 96.5 percent of all earth's water is salt water. identify the watery feature on earth that is made of freshwater rather than salt water. a) bay b) glacier c) ocean d) sea it is not incomplete this is the true question
Answers: 1
You know the right answer?
during a reaction, δh for reactants is −750 kj/mol and δh for products is 920 kj/mol. which statemen...
Questions in other subjects:

Mathematics, 26.09.2021 01:10



Mathematics, 26.09.2021 01:10

Biology, 26.09.2021 01:10

Social Studies, 26.09.2021 01:10




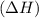 comes out to be positive.
comes out to be positive.


