Chemistry, 21.09.2019 14:50 caroline1484
5. given the cell notation cu(s)|cu2+(aq)||ag+(aq)|ag(s), what is the half-reaction that occurs at the anode?
cu2+(aq) + 2e–→ cu(s)
cu(s) → cu2+(aq) + 2e–
ag+(aq) + e–→ ag(s)
ag+(aq) + cu(s) → ag(s) + cu2+(aq)
6. what is the e0 for the spontaneous reaction when a ni2+/ni half-cell is joined to a cu2+/cu half-cell? e0cu= + 0.34 v = +0.34 v and e0ni= -0.26 v.
–0.08 v
+0.08 v
+0.60 v
–0.60 v

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 20:00, ahnorthcutt4965
Acm ruler with main graduations from 1 to 10 from left to right there are 10 secondary graduations between each of the main graduations there is a line that begins. at the left end of the ruler 10 secondary graduations to the left of the “1 main graduation the right end of the line ends on the eighth secondary graduation to the right of 3 how long is the line
Answers: 1

Chemistry, 22.06.2019 23:00, Kykebailey2356
Which of two curves exhibits exponential growth
Answers: 1


Chemistry, 23.06.2019 00:30, danielmartinez024m
Maya wrote if you step to describe how carbon circulates between the atmosphere and living organisms
Answers: 1
You know the right answer?
5. given the cell notation cu(s)|cu2+(aq)||ag+(aq)|ag(s), what is the half-reaction that occurs at t...
Questions in other subjects:

Mathematics, 27.09.2021 14:00


Mathematics, 27.09.2021 14:00



Mathematics, 27.09.2021 14:00

Mathematics, 27.09.2021 14:00

Mathematics, 27.09.2021 14:00


History, 27.09.2021 14:00


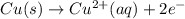

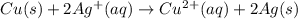
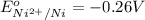

 of the reaction, we use the equation:
of the reaction, we use the equation: