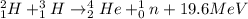The characteristics of two different types of reactions are shown below:
reaction a: an ato...

Chemistry, 21.10.2019 14:30 lindsayb2000
The characteristics of two different types of reactions are shown below:
reaction a: an atom loses electrons during the reaction.
reaction b: an atom loses protons and neutrons during the reaction.
which statement is true about the two reactions?
both reactions retain the identity of the elements.
both reactions change the identity of the elements.
reaction a produces more energy than reaction b.
reaction b produces more energy than reaction a.

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 08:40, jaueuxsn
Ageologist determines that a sample of a mineral can't be scratched by a steel nail but can be scratched by a masonry drill bit. based on this information, the sample mineral has to be softer than a. orthoclase. b. fluorite. c. apatite. d. corundum.
Answers: 2

Chemistry, 22.06.2019 09:00, 2024cynthiatercero
Which two scientific disciplines are bridged by pharmaceutical drugs? chemistry and forensics chemistry and medicine biology and forensics biology and criminology
Answers: 3

Chemistry, 22.06.2019 13:30, yasiroarafat12
How many moles is 14.5 cm^3 of platinum? the density of platinum is 21.45 g/cm^3.
Answers: 1

Chemistry, 22.06.2019 14:10, cameronbeaugh
13. a covalent bond between two atoms is likely to be polar if: a. one of the atoms is much more electronegative than the other. b. the two atoms are equally electronegative. c. the two atoms are of the same element. d. the bond is part of a tetrahedrally shaped molecule. e. one atom is an anion.
Answers: 1
You know the right answer?
Questions in other subjects:






Mathematics, 06.05.2020 23:59


English, 06.05.2020 23:59

Chemistry, 07.05.2020 00:00

 .
.