
Chemistry, 30.01.2020 07:50 wrerteteT2827
Consider the following reaction at equilibrium: 2co2 (g) ↔ 2co (g) + o2 (g) ∆h° = -514 kj le châtelier's principle predicts that removing o2(g) to the reaction container will consider the following reaction at equilibrium: 2co2 (g) 2co (g) + o2 (g) ∆h° = -514 kj le châtelier's principle predicts that removing o2(g) to the reaction container will decrease the value of the equilibrium constant increase the partial pressure of co increase the value of the equilibrium constant decrease the partial pressure of co increase the partial pressure of co2

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 21:00, tamikagoss22
Agas in a balloon at constant pressure has a volume of 160 ml at -125*c. what is its volume at 29.0*c?
Answers: 1

Chemistry, 22.06.2019 04:30, aleilyg2005
There is a single path for electrons. the current decreases when additional resistors are added. the current will be the same in each resistor. these statements best describe a(n) circuit.
Answers: 3

Chemistry, 22.06.2019 11:10, hannah2757
Which of the following shapes would represent a molecule with two bonded atoms and 3 lone pairs on only one of them , trigonal planar , bent , trigonal pyramidal , linear
Answers: 1

You know the right answer?
Consider the following reaction at equilibrium: 2co2 (g) ↔ 2co (g) + o2 (g) ∆h° = -514 kj le châtel...
Questions in other subjects:


Mathematics, 27.01.2020 21:31

Mathematics, 27.01.2020 21:31


History, 27.01.2020 21:31

Mathematics, 27.01.2020 21:31

History, 27.01.2020 21:31


Business, 27.01.2020 21:31

Mathematics, 27.01.2020 21:31

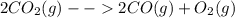 ∆H° = -514
∆H° = -514 

