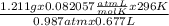Chemistry, 29.01.2020 03:59 svarner2001
Agas collected from a reaction has a mass of 1.211 g and occupies a volume of 0.677 l. the temperature in the laboratory is 296 k, and the air pressure is 0.987 atm. calculate the molar mass of the gas.
a) 23.7 g/mol
b) 44.0 g/mol
c) 58.5 g/mol
d) 82.3 g/mol

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 05:50, mrylenastewart
Fill in the coefficients that will balance the following reaction: a0cr2(so4)3 + a1agno3 -> a2cr(no3)3 + a3ag2so4
Answers: 1


Chemistry, 22.06.2019 23:30, billybob8514
To find the work done, the force exerted and distance moved are multiplied. a couch is moved twice before you are happy with its placement. the same force was used to move the couch both times. if more work is done the first time it is moved, what do you know about the distance it was moved? a) when more work was done, the couch was moved the same distance. b) when more work was done, the couch was moved less. c) when more work was done, the couch was moved further. d) when more work was done, the couch wasn't moved at all.
Answers: 1

Chemistry, 23.06.2019 01:00, kaykardash
Which polymers are most closely related? a. protein and nucleic acids b. cellulose and starch c. nucleic acids and starch d. nucleic acids and cellulose
Answers: 2
You know the right answer?
Agas collected from a reaction has a mass of 1.211 g and occupies a volume of 0.677 l. the temperatu...
Questions in other subjects:

History, 23.08.2019 05:40



English, 23.08.2019 05:40

Biology, 23.08.2019 05:40

Advanced Placement (AP), 23.08.2019 05:40

Mathematics, 23.08.2019 05:40

Social Studies, 23.08.2019 05:40


History, 23.08.2019 05:40

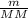 ) RT → MM =
) RT → MM =