
Chemistry, 28.09.2019 09:20 xmanavongrove55
Redox titrations are used to determine the amounts of oxidizing and reducing agents in solution. for example, a solution of hydrogen peroxide, h2o2, can be titrated against a solution of potassium permanganate, kmno4. the following equation represents the reaction: 2kmno4(aq)+h2o2(aq)+3h2so4(aq)→3o2( g)+2mnso4(aq)+k2so4(aq)+4h2o(l) a certain amount of hydrogen peroxide was dissolved in 100. ml of water and then titrated with 1.68 m kmno4. how much h2o2 was dissolved if the titration required 14.3 ml of the kmno4 solution?

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 22:30, kingteron5870
Ionic compounds are made of ions, and yet the overall charge of an ionic compound is neutral. why?
Answers: 1


Chemistry, 22.06.2019 15:30, elizabethprasad2
The reactions of photosynthesis occur in the of plant cell? a. mitochondria. b. lysosomes. c. chloroplasts. d. chlorophyll
Answers: 1

Chemistry, 22.06.2019 16:00, anaalashay
How will the volume of a gas be affected if the pressure is tripled, but the temperature remains the same?
Answers: 3
You know the right answer?
Redox titrations are used to determine the amounts of oxidizing and reducing agents in solution. for...
Questions in other subjects:

Mathematics, 30.04.2021 09:10




Spanish, 30.04.2021 09:10

Social Studies, 30.04.2021 09:10


English, 30.04.2021 09:10


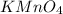 solution = 1.68 M
solution = 1.68 M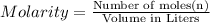
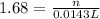
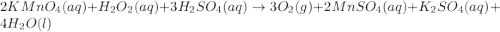
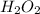 , then 0.0240 moles of KMnO_4 will react with :
, then 0.0240 moles of KMnO_4 will react with :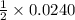 moles of
moles of 


