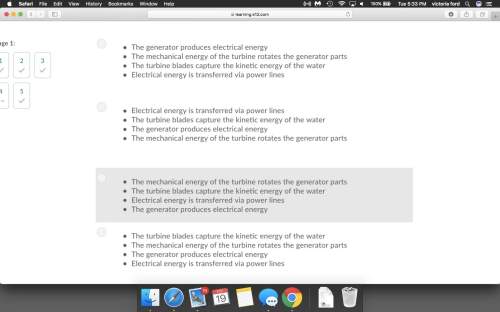
Ihave been having trouble figuring this out!
i'll mark whoever got this question answere...

Chemistry, 27.09.2019 16:30 jadeossowski3590
Ihave been having trouble figuring this out!
i'll mark whoever got this question answered correctly brainliest.

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 09:00, dante766
Achemist 16 drop copper metal from copper chloride solution. the chemist place is 0.50 g of aluminum foil in a solution containing 0.75 g of copper (ii) chloride. a single replacement reaction takes place. which statement explains the maximum amount of copper that the chemist can extract using this reaction?
Answers: 1

Chemistry, 22.06.2019 12:20, sindy35111
Consider the reaction of a(g) + b(g) + c(g) => d(g) for which the following data were obtained: experiment initial [a], mol/l initial [b], mol/l initial [c], mol/l initial rate, mol/l. s 1 0.0500 0.0500 0.0100 6.25 x 10^-3 2 0.100 0.0500 0.0100 2.50 x 10^-2 3 0.100 0.100 0.0100 1.00 x 10^-1 4 0.0500 0.0500 0.0200 6.25 x 10^-3 what is the rate law for the reaction?
Answers: 3

Chemistry, 22.06.2019 16:40, roderickhinton
The diagram below shows the movement of particles. what does this piece of evidence best support? the collision theory the maxwell-boltzmann distribution the effect of pressure on reaction rates the effect of temperature on reaction rates
Answers: 3

You know the right answer?
Questions in other subjects:

Mathematics, 17.03.2021 23:50

English, 17.03.2021 23:50

English, 17.03.2021 23:50

English, 17.03.2021 23:50




History, 17.03.2021 23:50

Mathematics, 17.03.2021 23:50




