
Chemistry, 19.01.2020 14:31 selena5713
Abuffer is prepared by adding 12.0g of ammonium chloride (nh4cl) to 250ml of 1.00 m nh3 solution? the ph is 9.3 write the net ionic equation for the reaction that occurs when a few drops of nitric acid (hno3) are added to the buffer.

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 09:00, dante766
Achemist 16 drop copper metal from copper chloride solution. the chemist place is 0.50 g of aluminum foil in a solution containing 0.75 g of copper (ii) chloride. a single replacement reaction takes place. which statement explains the maximum amount of copper that the chemist can extract using this reaction?
Answers: 1


Chemistry, 23.06.2019 03:00, amberskids2
You have a sample of a metal, the sample is exactly 6.02 x 1023atom, if the sample has a mass 55.85 what metal is your sample made of?
Answers: 2

You know the right answer?
Abuffer is prepared by adding 12.0g of ammonium chloride (nh4cl) to 250ml of 1.00 m nh3 solution? th...
Questions in other subjects:


Mathematics, 14.07.2019 14:40

Biology, 14.07.2019 14:40

English, 14.07.2019 14:40

Mathematics, 14.07.2019 14:40


Computers and Technology, 14.07.2019 14:40



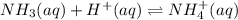
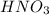 is a strong acid and nitrate ion is the spectator ion so it is not included in the net ionic equation.
is a strong acid and nitrate ion is the spectator ion so it is not included in the net ionic equation.

