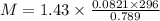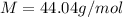Chemistry, 29.01.2020 04:43 kingken3400
The density of a gas is 1.43 g/l at a temperature of 23 ∘c and a pressure of 0.789 atm. calculate the molar mass of the gas.

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 02:30, BornAdopted21
Which piece of equipment would me most useful for measuring the volume of some water? a. pan balance b. graduated cylinder c. tweezers d. flask quick
Answers: 2

Chemistry, 22.06.2019 04:30, EinsteinBro
Which statement best describes the relationship between period and frequency of light waves? a) in wave b the period increases and the frequency decreases from wave a. b) in wave a the period increases and the frequency decreases from wave b. c) in wave b the period is shorter and the frequency is greater than in wave a. d) in wave a the period is shorter and the frequency is greater than in wave b.
Answers: 1


Chemistry, 22.06.2019 12:30, MrSavannahCat
Clyde and marilyn are riding a roller coaster. during which section(s) of the track is their potential energy converted to kinetic energy? a. from point b to point c only b. from point b to point d only c. from point a to point b only d. from point a to point b and from point c to point d
Answers: 1
You know the right answer?
The density of a gas is 1.43 g/l at a temperature of 23 ∘c and a pressure of 0.789 atm. calculate th...
Questions in other subjects:


Biology, 02.10.2019 02:30


Mathematics, 02.10.2019 02:30

Social Studies, 02.10.2019 02:30



SAT, 02.10.2019 02:30


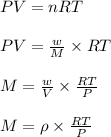
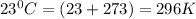
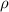 = density =1.43 g/ml
= density =1.43 g/ml