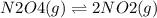Chemistry, 02.02.2020 10:44 kimlyn58p0wyn0
Which situation would cause the following equilibrium reaction to shift to the right? (1 point) n2o4 (g)two arrows stacked on top of each other. the top arrow points to the right. the bottom arrow points to the left. 2 no2
increase the pressure
add n2o4
add no2
decrease the volume

Answers: 1


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 19:30, Adrian12313
Estimate the molar mass of the gas that effuses at 1.6 times the effusion rate of carbon dioxide.
Answers: 1


Chemistry, 23.06.2019 05:40, girlchamp654
The independent variable in an experiment will be the variable that you o a) change ob) hold constant ng c) observe for changes
Answers: 2
You know the right answer?
Which situation would cause the following equilibrium reaction to shift to the right? (1 point) n2o...
Questions in other subjects:


Mathematics, 01.09.2019 20:50


History, 01.09.2019 20:50




Physics, 01.09.2019 20:50

Mathematics, 01.09.2019 20:50