
Chemistry, 10.10.2019 15:30 tdowling331
In a particular chemical reaction, the energy of the reactants is 30 kj and the energy of theproducts is 5 kj. the maximum energy of the system is 40 kj.
sketch a potential energy diagram for this reaction. make sure to label the energy of the reactants, the energy of the products, the activation energy, and the enthalpychange for the reaction.
what is the activation energy for this reaction? 40-30=10kjc.
what is the enthalpy change for this reaction? 5-30= -25kjd.

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 07:30, eburnhisel2023
The volume of helium in a blimp is 6.28 x 10^9 millimeters. the density of helium in the blimp is .1786 kilogram/meter^3. find the mass of the helium in the blimp.
Answers: 1

Chemistry, 22.06.2019 19:20, evansh78
Consider hydrogen in an excited state n = 5n=5 that emits photons to reach the ground state. there are various possible transitions other than straight to the ground state that can occur; for example, it can drop to the n = 3n=3 state followed by the n = 3n=3 to the ground state transition. which of the possible transitions will result in the emission of a photon in the visible region?
Answers: 3

Chemistry, 22.06.2019 20:30, demarcuswiseman
From the choices provided below, list the reagent(s) in order that will react with cyclopentanone to form the compound shown below.
Answers: 2
You know the right answer?
In a particular chemical reaction, the energy of the reactants is 30 kj and the energy of theproduct...
Questions in other subjects:






Mathematics, 15.04.2020 21:05


Mathematics, 15.04.2020 21:05

Mathematics, 15.04.2020 21:05

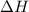 for the reaction comes out to be negative.
for the reaction comes out to be negative.


