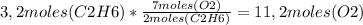Chemistry, 28.01.2020 19:10 SuperWoman9172
Read the given chemical reaction. c2h6 + o2 → co2 + h2o how many moles of o2 are required to react completely with 3.2 moles of c2h6? 3.5 moles of o2 6.5 moles of o2 10.4 moles of o2 11.2 moles of o2

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 09:30, mazielynn84
In 2002, the rare earth elements mine in mountain pass, california was closed because
Answers: 1

Chemistry, 22.06.2019 11:30, samantha9430
Determine the reaction and balance the following equations urgent due in the morning
Answers: 2

Chemistry, 22.06.2019 13:00, yaneiryx5476
Is 9 correct? and can someone me with 10? it’s due tomorrow, you
Answers: 1

Chemistry, 22.06.2019 20:20, carcon2019
Nitric acid can be formed in two steps from the atmospheric gases nitrogen and oxygen, plus hydrogen prepared by reforming natural gas. in the first step, nitrogen and hydrogen react to form ammonia: (g) (g) (g) in the second step, ammonia and oxygen react to form nitric acid and water: (g) (g) (g) (g) calculate the net change in enthalpy for the formation of one mole of nitric acid from nitrogen, hydrogen and oxygen from these reactions. round your answer to the nearest .
Answers: 3
You know the right answer?
Read the given chemical reaction. c2h6 + o2 → co2 + h2o how many moles of o2 are required to react c...
Questions in other subjects:









Social Studies, 20.08.2019 04:10