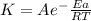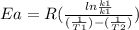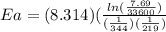Areaction is followed and found to have a rate constant of 3.36 × 104 m-1s-1 at 344 k and a rate constant of 7.69 m-1s-1 at 219 k. determine the activation energy for this reaction. a reaction is followed and found to have a rate constant of 3.36 × 104 m-1s-1 at 344 k and a rate constant of 7.69 m-1s-1 at 219 k. determine the activation energy for this reaction. 12.5 kj/mol 11.5 kj/mol 23.8 kj/mol 58.2 kj/mol 42.0 kj/mol

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 00:10, goodygoodgirlygirl
Which of the following best describes the formation of plasma?
Answers: 1


Chemistry, 22.06.2019 15:20, mydoggy152
Fossil fuels are organic compounds that are made from
Answers: 1

Chemistry, 22.06.2019 19:50, jakaylathomas11
A2.5% (by mass) solution concentration signifies that there is a 2.5 % (by mass) solution concentration signifies that there is blank of solute in every 100 g of solution. of solute in every 100 g of solution
Answers: 3
You know the right answer?
Areaction is followed and found to have a rate constant of 3.36 × 104 m-1s-1 at 344 k and a rate con...
Questions in other subjects:


English, 12.01.2021 17:30


Mathematics, 12.01.2021 17:30

Mathematics, 12.01.2021 17:30

Mathematics, 12.01.2021 17:30


Mathematics, 12.01.2021 17:30

Mathematics, 12.01.2021 17:30

English, 12.01.2021 17:30