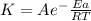Chemistry, 30.09.2019 22:00 GingerSnaps
The rate constant (k) for a reaction was measured as a function of temperature. a plot of lnk versus 1/t(in k) is linear and has a slope of −9.20×103 k . you may want to reference (pages 642 - 648) section 14.5 while completing this problem. part a calculate the activation energy for the reaction.

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 17:30, nique0808
What are the major products produced in the combustion of c10h22 under the following conditions? write balanced chemical equations for each. a. an excess of oxygen b. a slightly limited oxygen supply c. a very limited supply of oxygen d. the compound is burned in air
Answers: 2

Chemistry, 22.06.2019 12:00, kayla32213
Under normal conditions, describe how increasing the temperatures effects the solubility of a typical salt
Answers: 1

Chemistry, 22.06.2019 17:20, phanuel642
The small bags of silica gel you often see in a new shoe box are placed there to control humidity. despite its name, silica gel is a solid. it is a chemically inert, highly porous, amorphous form of sio2. because water vapor readily adsorbs onto the surface of silica gel, it acts as a desiccant. despite not knowing mechanistic details of the adsorption of water onto silica gel, from the information provided you should be able to make an educated guess about the thermodynamic characteristics of the process. predict the signs for δg, δh, and δs for the adsorption of water.
Answers: 2
You know the right answer?
The rate constant (k) for a reaction was measured as a function of temperature. a plot of lnk versus...
Questions in other subjects:

History, 25.07.2019 20:30




History, 25.07.2019 20:30

Health, 25.07.2019 20:30

Advanced Placement (AP), 25.07.2019 20:30

Social Studies, 25.07.2019 20:30